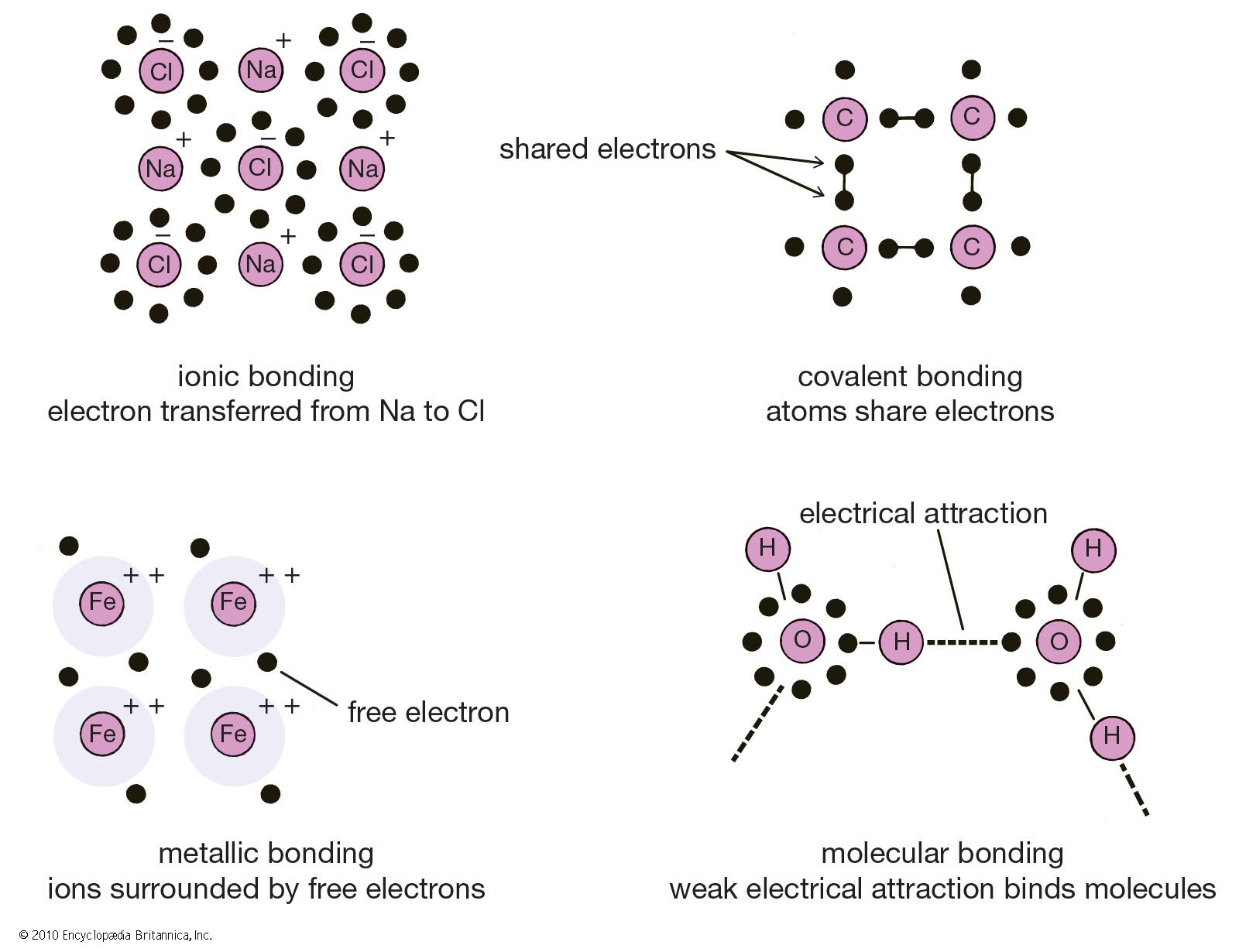
Why Are Ionic Bonds Good Conductors . ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. Ionic compounds are hard and brittle. a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. ionic compounds have high melting points. An ionic bond is the strongest type of chemical bond,. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. ionic compounds form when atoms connect to one another by ionic bonds. But when they're dissociated in a solution or through. in their bonded, solid states, molecules like salt don’t conduct electricity. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. This type of bonding leads to the formation of two.
from www.compoundworksheets.com
an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. But when they're dissociated in a solution or through. in their bonded, solid states, molecules like salt don’t conduct electricity. This type of bonding leads to the formation of two. An ionic bond is the strongest type of chemical bond,. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. ionic compounds have high melting points. ionic compounds form when atoms connect to one another by ionic bonds. Ionic compounds are hard and brittle.
Chemical Bonding Ionic And Covalent Compounds Britannica
Why Are Ionic Bonds Good Conductors in their bonded, solid states, molecules like salt don’t conduct electricity. in their bonded, solid states, molecules like salt don’t conduct electricity. This type of bonding leads to the formation of two. a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. Ionic compounds are hard and brittle. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. ionic compounds have high melting points. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. ionic compounds form when atoms connect to one another by ionic bonds. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. An ionic bond is the strongest type of chemical bond,. But when they're dissociated in a solution or through.
From slidetodoc.com
MYP Chemistry Ionic Bonding and Ionic Compounds International Why Are Ionic Bonds Good Conductors ionic compounds have high melting points. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. ionic compounds form when atoms connect to one another by ionic bonds. in. Why Are Ionic Bonds Good Conductors.
From www.slideserve.com
PPT Ionic Bonding Part I PowerPoint Presentation, free download ID Why Are Ionic Bonds Good Conductors This type of bonding leads to the formation of two. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. Ionic compounds are hard and brittle. But when they're dissociated in a solution or through. ionic compounds have high melting points. An ionic bond is the strongest type of chemical bond,. . Why Are Ionic Bonds Good Conductors.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Are Ionic Bonds Good Conductors But when they're dissociated in a solution or through. Ionic compounds are hard and brittle. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds have high melting points. An ionic bond is the strongest type. Why Are Ionic Bonds Good Conductors.
From www.worksheetsplanet.com
What is an Ionic Bond? Why Are Ionic Bonds Good Conductors An ionic bond is the strongest type of chemical bond,. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. in their bonded, solid states, molecules like salt don’t conduct electricity. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. an. Why Are Ionic Bonds Good Conductors.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Why Are Ionic Bonds Good Conductors ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. This type of bonding leads to the formation of two. in their bonded, solid states, molecules like salt don’t conduct electricity. ionic compounds form when atoms connect to one another by ionic bonds. But when they're dissociated in a solution or. Why Are Ionic Bonds Good Conductors.
From sciencenotes.org
Ionic Bond Definition and Examples Why Are Ionic Bonds Good Conductors a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. This type of bonding leads to the formation of two. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. in their bonded, solid states, molecules like salt don’t conduct electricity.. Why Are Ionic Bonds Good Conductors.
From www.thoughtco.com
Examples of Ionic Bonds and Ionic Compounds Why Are Ionic Bonds Good Conductors ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. But when they're dissociated in a solution or through. This type of bonding leads to the formation of two. Ionic compounds are hard and brittle. in their bonded, solid states, molecules like salt don’t conduct electricity. a variety of crystalline phosphates. Why Are Ionic Bonds Good Conductors.
From thescienceandmathszone.com
Ionic Bonding The Science and Maths Zone Why Are Ionic Bonds Good Conductors in their bonded, solid states, molecules like salt don’t conduct electricity. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. This type of bonding leads to the formation of two. But when they're dissociated in a solution or through. an ionic bond is the bond formed by the complete transfer. Why Are Ionic Bonds Good Conductors.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Ionic Bonds Good Conductors ionic compounds have high melting points. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. An ionic bond is the strongest type of chemical bond,. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. in their bonded, solid states, molecules like. Why Are Ionic Bonds Good Conductors.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Ionic Bonds Good Conductors Ionic compounds are hard and brittle. But when they're dissociated in a solution or through. in their bonded, solid states, molecules like salt don’t conduct electricity. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. This type of bonding leads to the formation of two. ionic compounds have. Why Are Ionic Bonds Good Conductors.
From dxoejbyrw.blob.core.windows.net
Why Are Ions Good Conductors Of Electricity at Nellie Ball blog Why Are Ionic Bonds Good Conductors ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. in their bonded, solid states, molecules like salt don’t conduct electricity. An ionic bond is the strongest type of chemical bond,. ionic compounds have high melting points. This type of bonding leads to the formation of two. ionic. Why Are Ionic Bonds Good Conductors.
From slideplayer.com
Bonding Ionic Bonding. ppt download Why Are Ionic Bonds Good Conductors in their bonded, solid states, molecules like salt don’t conduct electricity. But when they're dissociated in a solution or through. ionic compounds form when atoms connect to one another by ionic bonds. This type of bonding leads to the formation of two. ionic compounds have high melting points. ionic compounds are good conductors of electricity when. Why Are Ionic Bonds Good Conductors.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Why Are Ionic Bonds Good Conductors Ionic compounds are hard and brittle. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. in their bonded, solid states, molecules like salt don’t conduct electricity. This type of bonding leads to the formation of two. ionic solids are also poor conductors of electricity for the same reason—the strength. Why Are Ionic Bonds Good Conductors.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542 Why Are Ionic Bonds Good Conductors a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. This type of. Why Are Ionic Bonds Good Conductors.
From www.tumblr.com
ionicbond on Tumblr Why Are Ionic Bonds Good Conductors an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. But when they're dissociated in a solution or through. An ionic bond is the strongest type of chemical bond,. ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. This type of bonding. Why Are Ionic Bonds Good Conductors.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID4493576 Why Are Ionic Bonds Good Conductors a variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x. An ionic bond is the strongest type of chemical bond,. ionic compounds form when atoms connect to one another by ionic bonds. But when they're dissociated in a solution or through. Ionic compounds are hard and brittle. This. Why Are Ionic Bonds Good Conductors.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Why Are Ionic Bonds Good Conductors ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. This type of bonding leads to the formation of two. in their bonded, solid states, molecules like salt don’t conduct electricity. But. Why Are Ionic Bonds Good Conductors.
From www.compoundworksheets.com
Chemical Bonding Ionic And Covalent Compounds Britannica Why Are Ionic Bonds Good Conductors This type of bonding leads to the formation of two. an ionic bond is the bond formed by the complete transfer of valence electron to attain stability. ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds. An ionic bond is the strongest type of chemical bond,. a variety of crystalline. Why Are Ionic Bonds Good Conductors.